Jupiter Neurosciences, Inc. Announces Publication in Journal of Alzheimer’s Disease Highlighting Use of JOTROL™
Preclinical proof of concept demonstrated in a disease model
JUPITER, Florida. and BOSTON, June 14, 2022 /PRNewswire/ — Jupiter Neurosciences, Inc. (“Jupiter” or the “Company”), announced today the publication in the Journal of Alzheimer’s Disease of “JOTROL™, a Novel Formulation of Resveratrol, Shows Beneficial Effects in the 3xTg-AD Mouse Model”. This research, carried out in collaboration with the University of Miami The Miller School of Medicine demonstrated that a new oral formulation of resveratrol, JOTROL™, was able to provide therapeutically viable levels of resveratrol in a mouse model of Alzheimer’s disease (AD). The results showed that JOTROL™ significantly increased bioavailability compared to unformulated resveratrol and that the treatment led to changes in the expression of AD-related genes, as well as changes in the levels of inflammatory genes and cytokines. JOTROL™ may be effective as prophylaxis and/or treatment of AD through increased expression and/or activation of neuroprotective genes, suppression of pro-inflammatory genes, and regulation of central and peripheral cytokine levels. “This study demonstrated the much improved bioavailability and activity of JOTROL™ compared to unformulated resveratrol in an animal model of Alzheimer’s disease with prolonged drug exposure,” said lead author Prof. Claes WahlestedtAssociate Dean for Therapeutic Innovation, Department of Psychiatry and Behavioral Health at University of Miami Miller School of Medicine.”
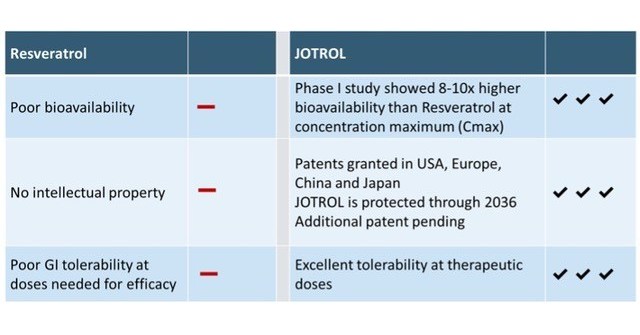
Comparison of JOTROL™ product differentiation.
About JOTROL™
JOTROL™, the Company’s unique, patented platform product, is an enhanced resveratrol formulation designed to safely deliver therapeutically relevant levels of resveratrol. In a first-in-man phase I trial, JOTROL™ was administered at increasing doses to assess safety, tolerability and pharmacokinetics. JOTROL™ was found to be safe and well tolerated at all doses administered and achieved target blood plasma levels 8-10 times higher than naïve resveratrol administered in historical clinical trials. The study was funded by a grant from the National Institute on Aging (NIA), titled Safety and Pharmacokinetics of JOTROL for Alzheimer’s Disease. Resveratrol can cross the blood-brain barrier and has been shown to have positive effects on oxidative stress, inflammation, and mitochondrial function in patients with Friedreich’s ataxia (FA) and Alzheimer’s disease (AD).
About Jupiter Neurosciences, Inc.
Jupiter Neurosciences, Inc. is a clinical-stage pharmaceutical company specializing in the treatment of CNS disorders and rare diseases. The Company’s platform product, JOTROL™, offers potential therapeutic benefit for most central nervous system diseases such as Alzheimer’s disease, ataxias and metabolic disorders such as lysosomal storage disorders and mitochondrial diseases. Data from human clinical trials show benefits in multiple indications and the FDA has accepted an initial Investigational New Drug (IND) application for JOTROL™. More information can be found on the company’s website www.jupiterneurosciences.com.
Forward-looking statements
This press release may contain certain statements regarding future results that are forward-looking statements. It is possible that the Company’s actual results and financial condition may differ, perhaps materially, from the anticipated results and financial condition indicated in these forward-looking statements, depending on factors, including whether results obtained in preclinical studies and non-clinical and clinical trials will be indicative of results obtained in future clinical trials; whether preliminary or interim results of a clinical trial will be indicative of the final results of the trial; the size of potential markets for the Company’s drug candidates and its ability to serve those markets; and the Company’s current and future capital needs and its ability to raise additional funds to meet its capital needs. All forward-looking statements included in this press release are made only as of the date of this press release, and we undertake no obligation to publicly update or correct any forward-looking statements to reflect events or circumstances that occur subsequently or of which we become aware subsequently.
contacts:
Investor Relations
Alison SilvaPresident & CBO
Jupiter Neurosciences, Inc.
[email protected]
Media Relations
Alex Rosen, General Manager
Jupiter Neurosciences, Inc.
561-406-6154
[email protected]
SOURCEJupiter Neurosciences, Inc.







