Privo Technologies, Inc. Announces Publication in Nature Communications
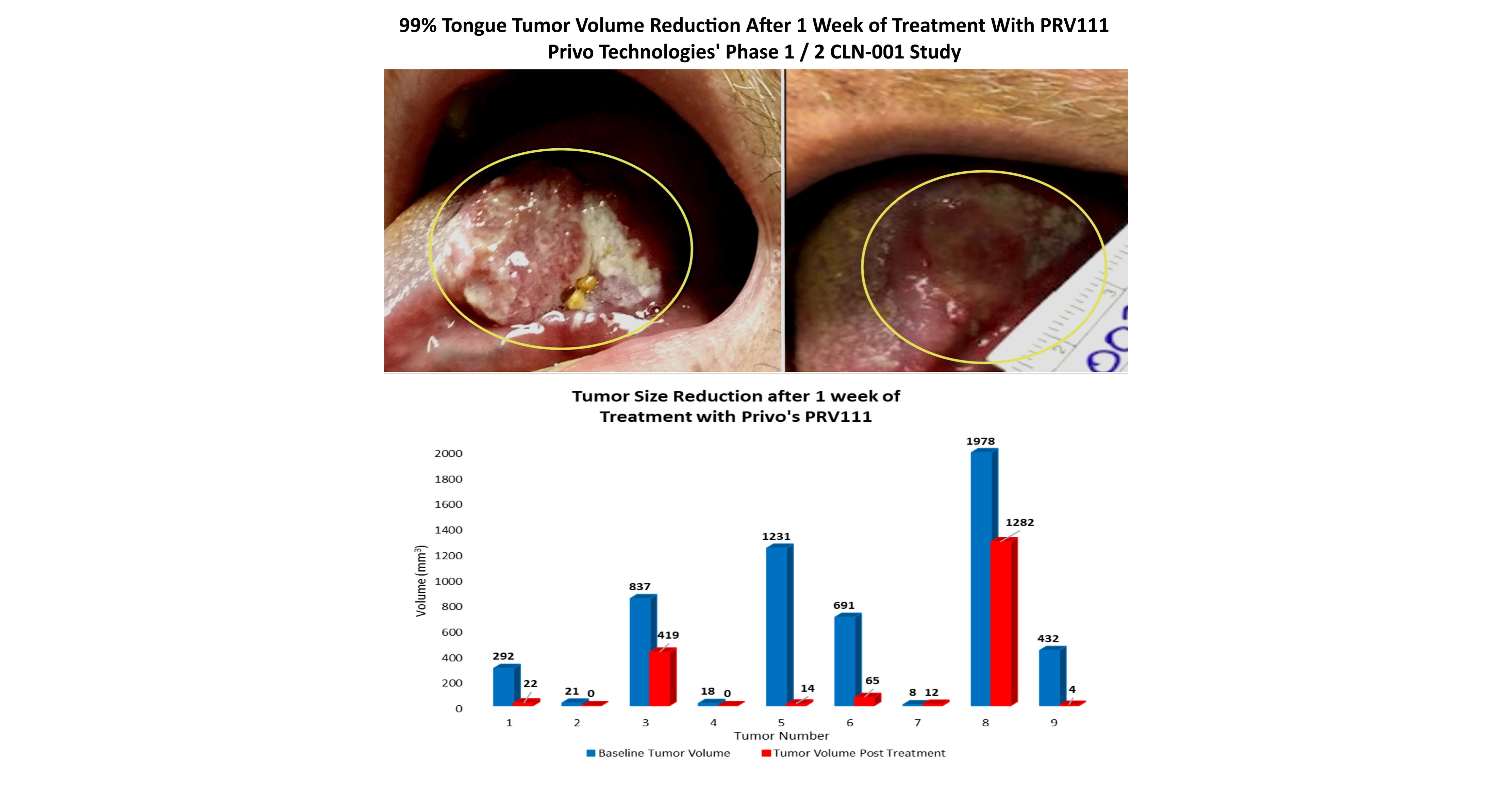
PEABODY, Mass., August 24, 2022 /PRNewswire/ — Privo Technologies, Inc. (“Privo”), a Phase 3 clinical-stage biopharmaceutical company that has designed and developed a nanotechnology drug delivery platform to safely deliver highly potent drugs from locoregional manner, announced that Nature Communication published the results of the evaluation of efficacy and safety data collected using PRV111 in animal models of oral cancer and clinical trials in patients with oral squamous cell carcinoma (OCSCC) .
The article titled, “Nano-engineered topical transmucosal cisplatin delivery system induces anti-tumor response in animal models and oral cancer patientsdetails the observed therapeutic benefit of topical delivery, via the PRV platform, of high concentrations of cisplatin to the tumor and regional lymph nodes while avoiding systemic circulation. high-level peer-reviewed like Nature Communications. These data highlight the promise of targeted and local chemotherapy treatment and validate the superior mechanism and design of the PRV platform and its lead derivative PRV111. These data are a critical step to enter a phase 3 clinical trial for patients with carcinoma in situ of the oral cavity as well as a springboard to explore the use of the platform in other mucosal cancers such as cancers of the lung, cervix, vagina, rectum. , and anal cancers,” said Manijeh Goldberg, PhD, Founder and CEO of Privo. Dr. Goldberg is the first author of the article, which was published in collaboration with Dr. Nishant AgrawalMD FACS, and Dr. Evgeny Izumchenko, PhD, at University of Chicago, Department of Surgery and Department of Medicine, respectively. Dr. Goldberg, Dr. Agrawal and Dr. Izumchenko are the corresponding authors.
“The data in this paper validates our platform for nano-encapsulating highly potent drugs while providing controlled delivery and release. Privo is excited to add to the growing body of scientific evidence demonstrating that encapsulation for the delivery localization of highly toxic chemotherapies and other drugs may unlock therapeutic benefits, disrupting the targeted drug delivery industry both topically and intraoperatively,” said Dr. Goldberg. “We are grateful to our sites clinics and their research teams for their participation in this study, and of course indebted to our patients and the oral cancer community for their support of our technology.” Enrollment sites included the School of Dental Medicine from the UT Health Sciences Center and Baylor College of Medicine in Houston, TX; University of Cincinnati Cancer Institute of Cincinnati, Ohio; Advanced ENT and allergy in Louisville, Kentucky; Ben Taub Hospital and Hermann Memorial Hospital in Houston, TX.
“Oral cancer treatment can involve life-changing surgery that can impact function. The data presented on Privo’s PRV111 is an important step in the approval process for what will be a transformative product that has the potential to change the standard of care,” said Dr. Nishant Agrawal. Dr. Agrawal is Head of the Department of Otolaryngology-Head and Neck Surgery and Co-Head of the Department of Head and Neck Surgical Oncology at University of Chicago.
The complete article is available in hard copy and in digital format which can be consulted via the following link: (https://www.nature.com/articles/s41467-022-31859-3).
Nature Communication is an open access, peer-reviewed, multidisciplinary journal dedicated to publishing high-quality research in all areas of biological, health, physical, chemical, and earth sciences.
About Privo Technologies, Inc.
Privo Technologies, Inc. (“Privo”) is a Phase 3 clinical-stage biopharmaceutical company engaged in the development of innovative therapies designed to eliminate cancers and prevent recurrences. Privo aims to provide better and more accessible treatment options to diverse patient populations around the world with the potential to transform the standard of care for the treatment of solid tumors. Privo is headquartered in Peabodya suburb of Boston, MA.
Privo’s lead asset, PRV111, has been shown to be effective in patients with head and neck cancer at multiple hospitals across the United States in a Phase I/II clinical study of safety and efficacy, dramatically reducing tumor volume without any systemic toxicity. For more information about Privo Technologies, Inc., please visit www.privotechnologies.com.
Media Contact: [email protected]
SOURCE Privo Technologies







